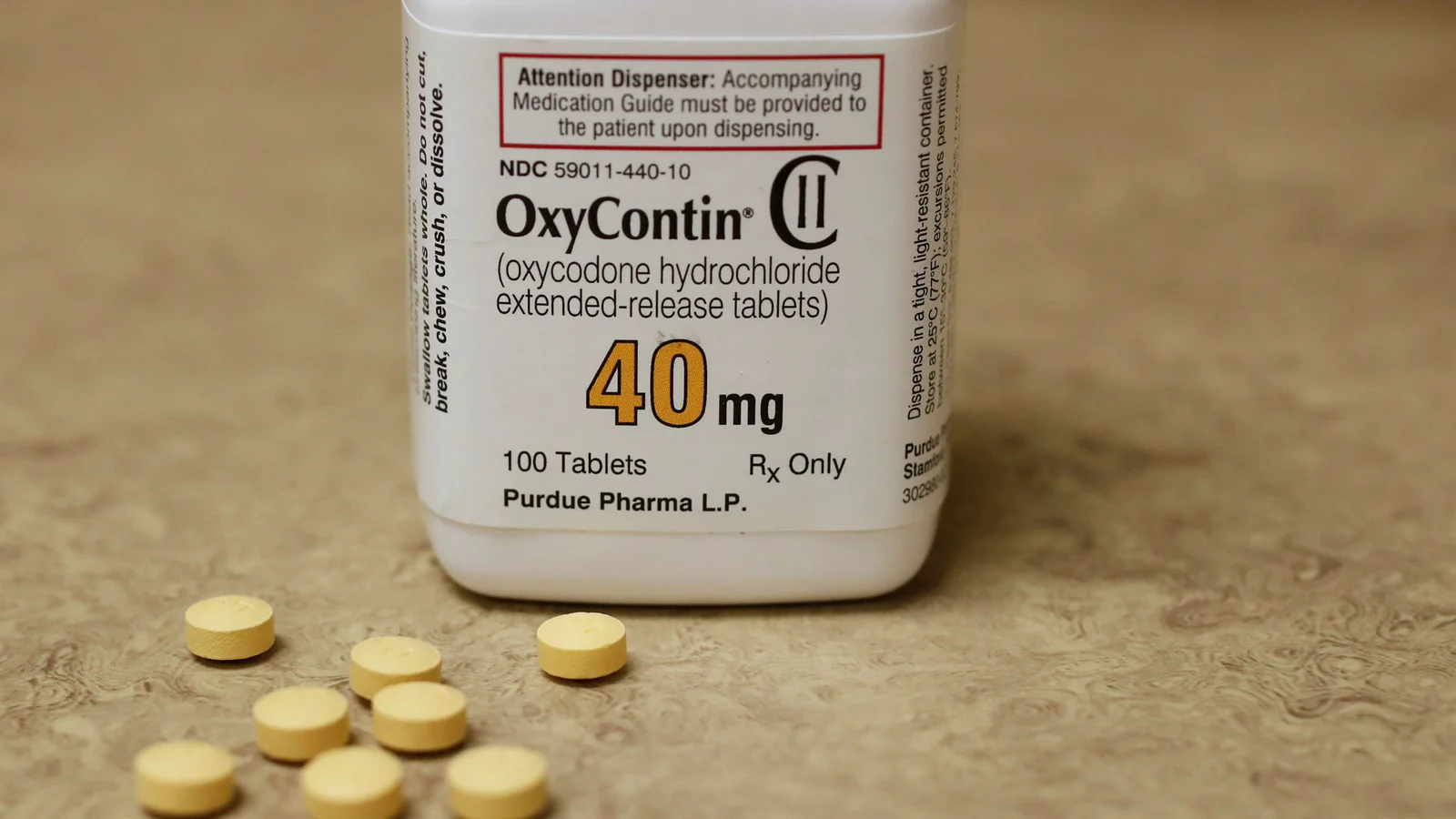
oxycontin 40mg
$200.00 – $1,500.00Price range: $200.00 through $1,500.00
Please use the sharing tools found via the share button at the top or side of articles. Copying articles to share with others is a breach of FT.com T&Cs and Copyright Policy.
Please use the sharing tools found via the share button at the top or side of articles. Copying articles to share with others is a breach of FT.com T&Cs and Copyright Policy. @ft.com to buy additional rights. Subscribers may share up to 10 or 20 articles per month using the gift article service. More information can be found here.
Purdue Pharma has announced that the US drug regulator plans to fast track its new treatment for suspected opioid overdoses, pledging to try to address the opioid crisis it is blamed for helping to stoke. The embattled maker of OxyContin, owned by the Sackler family, said its nalmefene hydrochloride injection had been granted fast-track status by the Food and Drug Administration, setting it up for an expedited review. The injection has a longer-lasting effect than naloxone, the current treatment for opioid overdoses, the company said. The FDA did not respond to a request for comment. Purdue hopes the product could be available in the next two years. Purdue says it will not profit from the new treatment if it is approved by the FDA, and said that it was exploring ways to ensure it would get to the patients that needed it the most. Craig Landau, chief executive of Purdue, told the Financial Times that Purdue had been committed for more than 20 years to addressing problems associated with opioids. “We have been very serious for a long time mitigating known risks associated with our prescription products,” he said. The majority of drug overdose deaths in the US are caused by opioids, with an average of 130 deaths a day, according to the Center for Disease Control and Prevention. Addiction to both prescribed and illegal opioids has ravaged communities across the US, to such an extent that people in public service jobs such as teachers and librarians are being trained to administer naloxone. The maker of naloxone, Kaleo, was criticised last year by lawmakers in the US for the high price of the drug. The company responded by announcing it would put a generic version on the market at a much lower cost this year. Purdue, meanwhile, faces thousands of lawsuits and investigations by several state attorneys-general over its alleged aggressive sales tactics of OxyContin, a potent opioid painkiller, and is considering filing for bankruptcy protection. The Sackler family, best known before the opioid crisis as philanthropists, also owned a second opioid maker, the FT revealed last year. Recommended FT SeriesOpioids and the Sackler family How Purdue’s ‘one-two’ punch fuelled the market for opioids Dr Landau told the FT on Wednesday that Purdue saw bankruptcy as one of many options — but that he could not put a probability on how likely it is. “We’re exploring and we are preparing for any number of eventualities and options, which is the responsible thing to do as the leader of this company,” he said. “We need to be prepared for given what may come in the weeks and months ahead, as we are involved in significant litigation.” The company hired the legal firm Davis Polk & Wardell for advice on
| ssss | 100 Pills, 300 Pills, 500 Pills, 1000 Pills |
|---|
Be the first to review “oxycontin 40mg” Cancel reply
You must be logged in to post a review.
Related products
Pills
Pills
Pills
Pills











Reviews
There are no reviews yet.